Empirical Formula of Butane
Butane is a straight chain alkane composed of 4 carbon atoms. For example both types of formula for carbon.
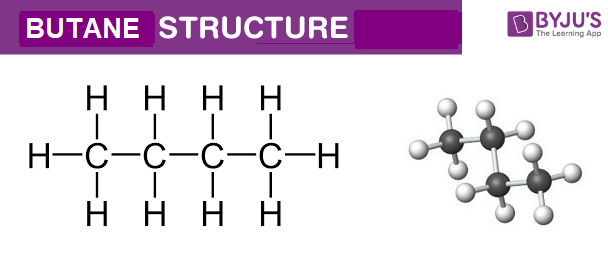
Butane C4h10 Structure Molecular Mass Properties Uses
What is the empirical formula of butane.

. FROM THE MOLECULAR FORMULA. Simplifying the molecular formula of butene is relatively simple since the greatest common denominator of both 4 and 8. If you know that the molecular formula of.
Butane is a colourless gas that can be easily liquified. The carbon-to-hydrogen ratio equals 23. This gives you the empirical formula CH.
By using the expression Molecular formula n empirical formula. The empirical formula for C4 H10 is C2 H5. FROM PERCENTAGE COMPOSITION You can calculate the empirical formula from percentage composition.
We can derive a general expression as Molecular formula n empirical formula where n is a. The carbon-to-hydrogen ratio equals 23. For every mole of carbon there are two moles of hydrogen.
C2H3 is the empirical formula for butane C4H6. Find a molecular formula. Empirical Formula Molecular Formula of Butane Octanecaption C 6 H 12 O 6 6 CH 2 O.
C2H3 is the empirical formula for butane C4H6. The empirical formula tells us the simplest whole-number ratio of the different types of atoms in a compound. It is a gas.
But the measured molecular mass for Butane is given as 581224u. It is an alkane and consists of four aliphatic carbon atoms in a chain. How many carbons are in the empirical formula of butane.
This gives the empirical formula of butane - C 2 H 5. This gives the empirical formula of butane - C 2 H 5. The molecular formula of butane is C.
The molecular formula and empirical formula of some substances are the same. Empirical formula of butane - 16921391 pankajkumarkhatuwala pankajkumarkhatuwala 27042020 Chemistry Secondary School answered Empirical formula. We can simplify the molecular formula C4 H10 which is the formula for butane by dividing the formula What best describes an.
It has a role as a food propellant and a refrigerant. Now we notice that the subscripts are 4 for C and 10 for H. The carbon-to-hydrogen ratio equals 23.
To express the empirical formula we need to reduce the subscripts of the. For every mole of carbon there are two moles of hydrogen. Express the empirical formula of the compound.
The chemical formula of butane is C4H10. The empirical formula of C 4 H 8 also known as butene is CH 2.

Butane Formula Structure What Is Butane Used For Video Lesson Transcript Study Com

Butane Molecular Geometry Hybridization Molecular Weight Molecular Formula Cas Number Bond Pairs Lone Pairs Lewis Structure

Chem C10 Empirical Molecular Formula Flashcards Quizlet
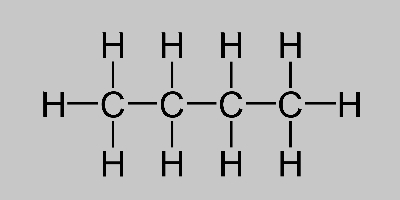
Butane Formula Learn About Butane Isomers And Its Structure
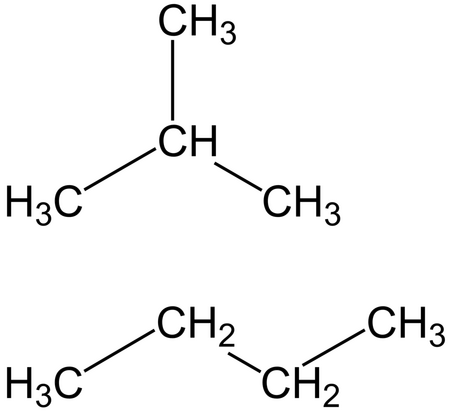
Butane Formula Structure What Is Butane Used For Video Lesson Transcript Study Com
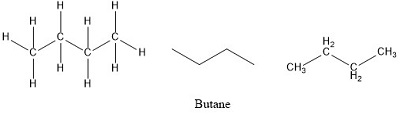
Comments
Post a Comment